PROIN ER™
(phenylpropanolamine hydrochloride extended-release tablets)
USES
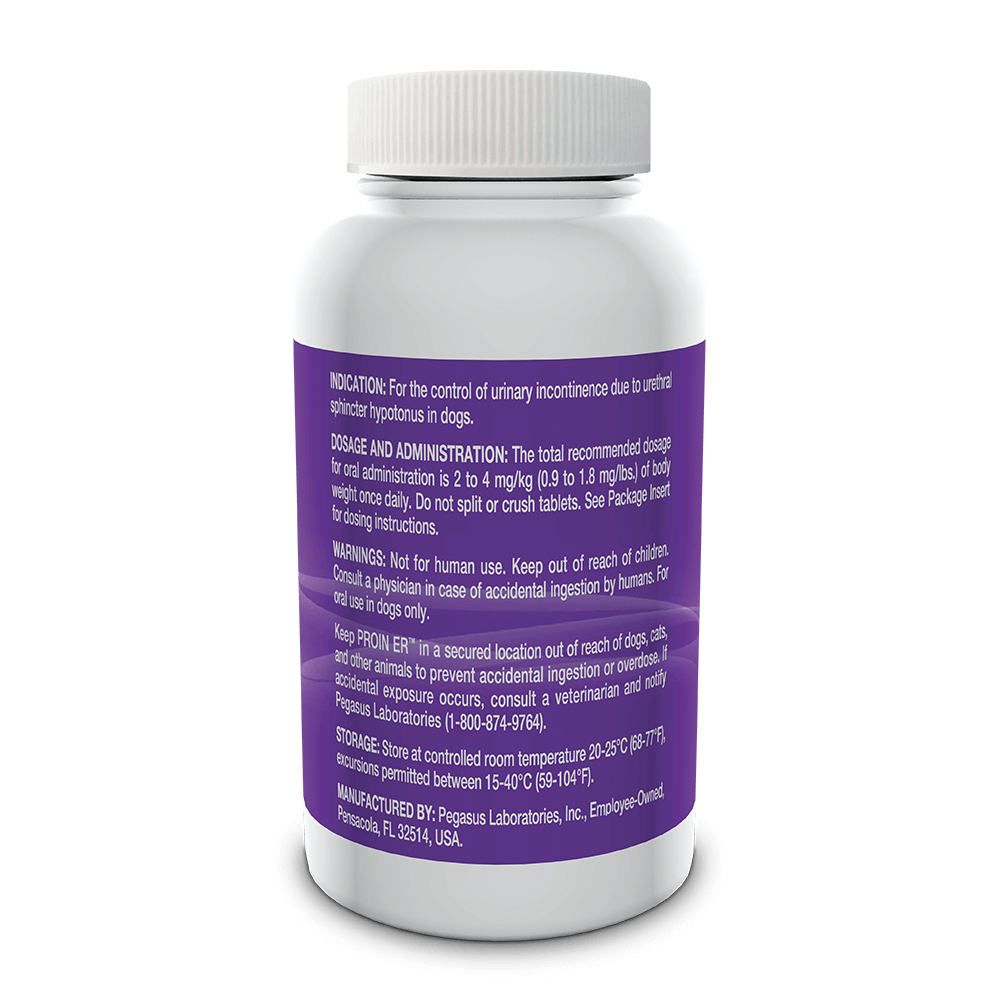
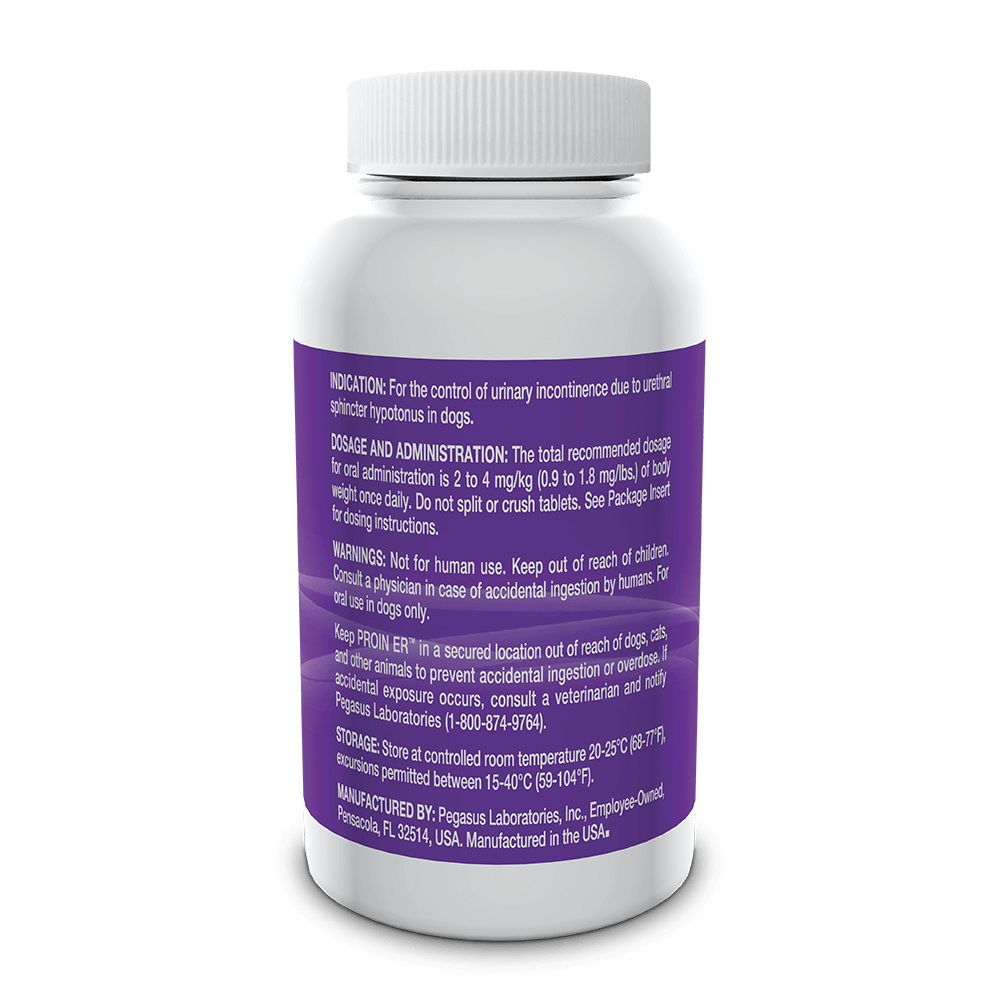
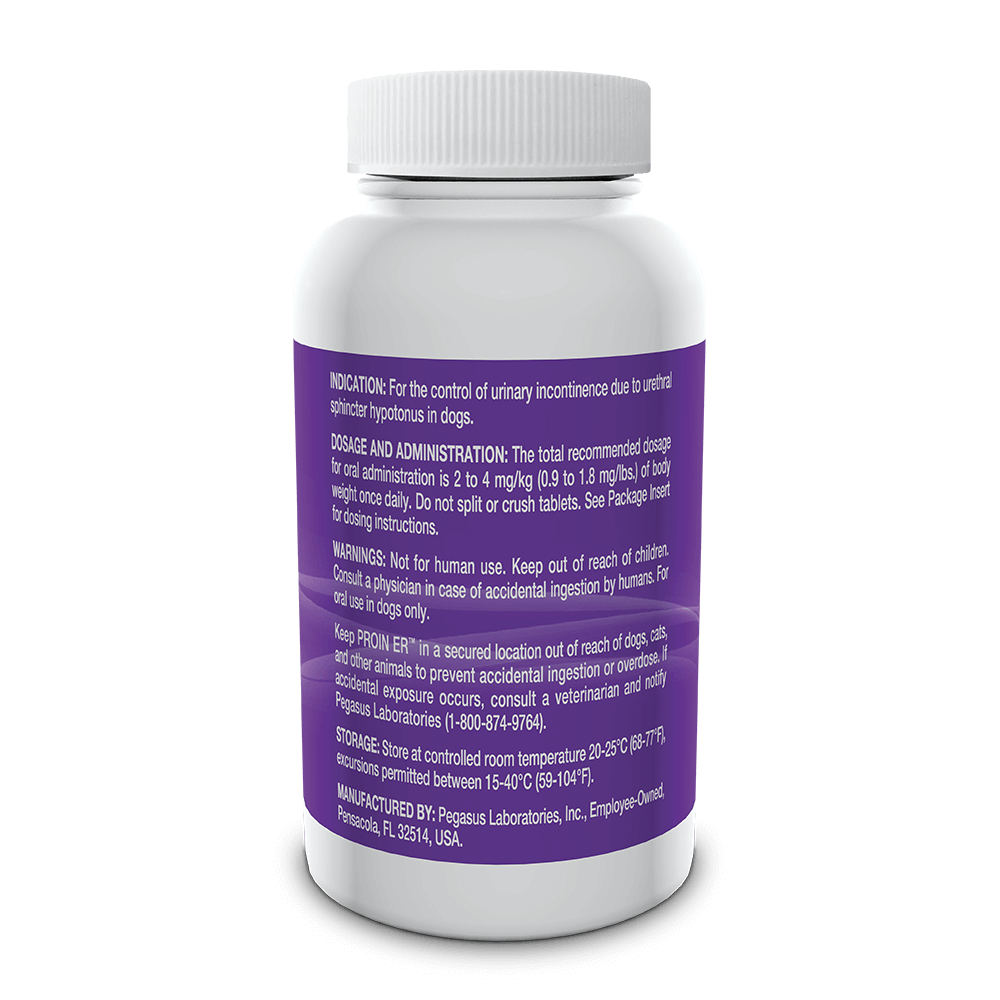
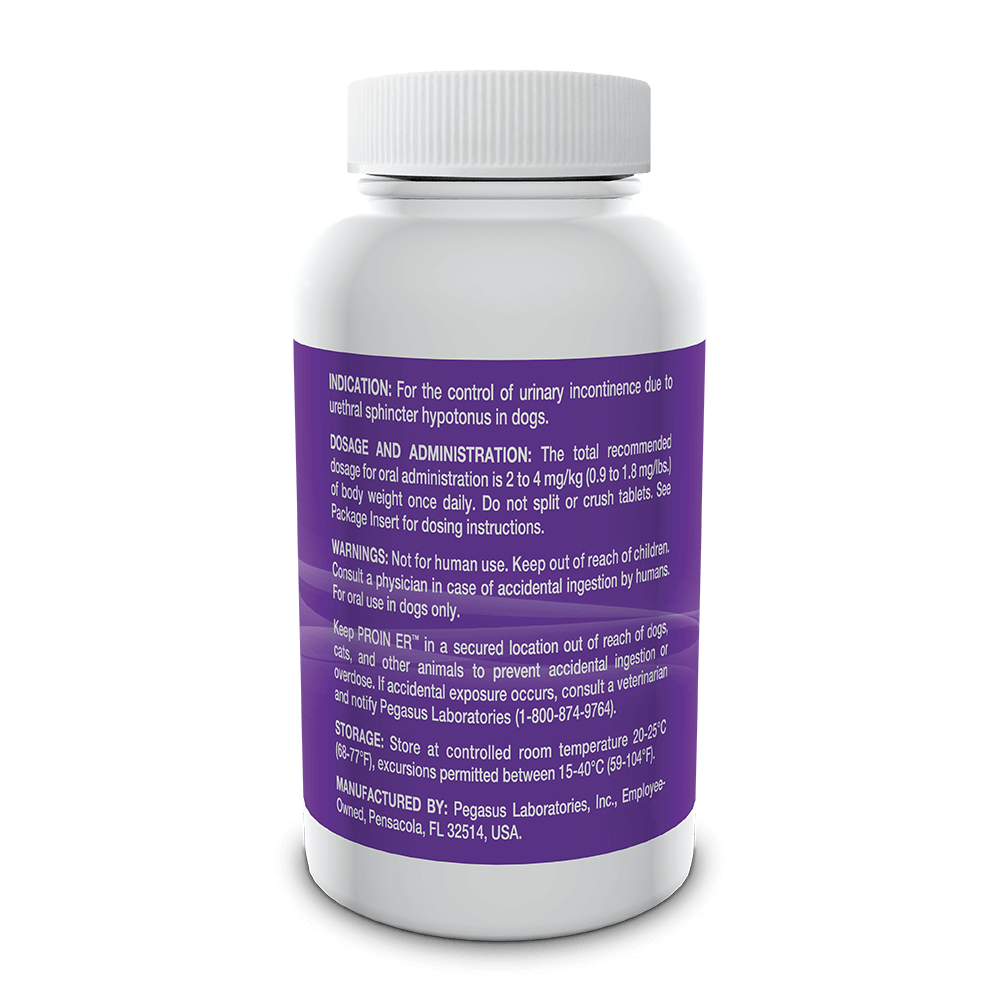
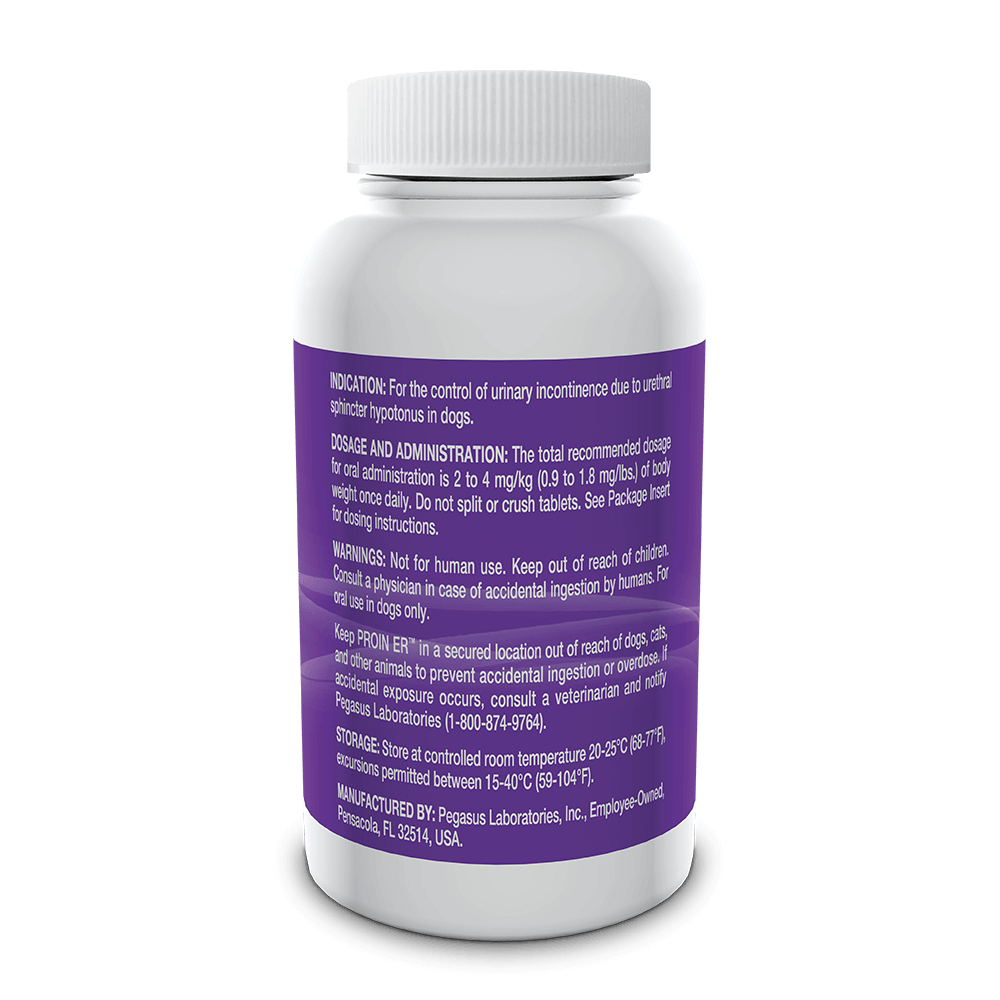
Indicated for the control of urinary incontinence due to urethral sphincter hypotonus in dogs.
Federal law restricts this drug to use by or on the order of a licensed veterinarian.
See full prescribing information for complete dosing and administration instructions.
| Item # | Product Name | Supplied |
|---|---|---|
| 30034457 | PROIN ER™ 18 mg Tablets | 30 ct. bottle |
| 30034444 | PROIN ER™ 18 mg Tablets | 90 ct. bottle |
| 30034557 | PROIN ER™ 38 mg Tablets | 30 ct. bottle |
| 30034544 | PROIN ER™ 38 mg Tablets | 90 ct. bottle |
| 30034657 | PROIN ER™ 74 mg Tablets | 30 ct. bottle |
| 30034644 | PROIN ER™ 74 mg Tablets | 90 ct. bottle |
| 30034757 | PROIN ER™ 145 mg Tablets | 30 ct. bottle |
| 30034744 | PROIN ER™ 145 mg Tablets | 90 ct. bottle |
ONLINE RESOURCES
PRODUCT INFORMATION
Active Ingredient(s): phenylpropanolamine hydrochloride
IMPORTANT SAFETY INFORMATION:
The most commonly reported side effects were vomiting, loss of appetite, diarrhea, excessive salivation, agitation, tiredness, vocalization, confusion, increased water consumption, weight loss, weakness, fever, panting, and reversible changes in skin color (flushing or bright pink). Abnormal gait, seizures or tremors, as well as liver enzyme elevations, kidney failure, blood in urine and urine retention have been reported. In some cases, death, including euthanasia, has been reported. Sudden death was sometimes preceded by vocalization or collapse.
Instances of dogs chewing through closed vials of PROIN® and eating the vial contents have been reported, in some cases resulting in overdose. Keep the product in a secured storage area out of the reach of pets in order to prevent accidental ingestion or overdose, as dogs may willingly consume more than the recommended dosage of PROIN ER™ tablets. Contact your veterinarian immediately if the dog ingests more tablets than prescribed or if other pets ingest PROIN ER™ tablets.
PROIN ER™ may cause elevated blood pressure and should be used with caution in dogs with pre-existing heart disease, high blood pressure, liver disease, kidney insufficiency, diabetes, glaucoma, and other conditions associated with high blood pressure.
Dogs may transition from PROIN® Chewable Tablets to PROIN ER™ without a break in administration. However, do not alternate PROIN ER™ with PROIN® Chewable Tablets because effectiveness and safety of interchangeable use has not been evaluated.
The safe use of PROIN ER™ in dogs used for breeding purposes, during pregnancy or in lactating bitches, has not been evaluated. Contact your veterinarian if you notice restlessness or irritability, loss of appetite, the incontinence persists or worsens or any other unusual signs. See prescribing information for complete details regarding adverse events, warning and precautions.
WARNINGS:
For oral use in dogs only. Not for human use. Keep out of the reach of children. Consult with a physician in case of accidental ingestion by humans. Keep PROIN ER™ in a secured location out of the reach of dogs, cats and other animals to prevent accidental ingestion or overdose.
PROIN ER™ may cause increased thirst; therefore provide ample fresh water. Do not split or crush tablets.
Due to the nature of the active pharmaceutical ingredient, some states may have additional legal requirements for the prescribing, sale and purchase of PROIN ER™.